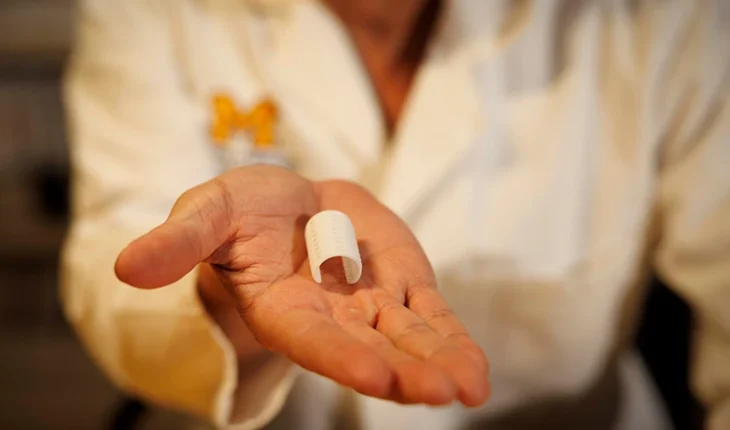
3D printed tracheobronchial splint to be implanted in at least 35 infants and children.
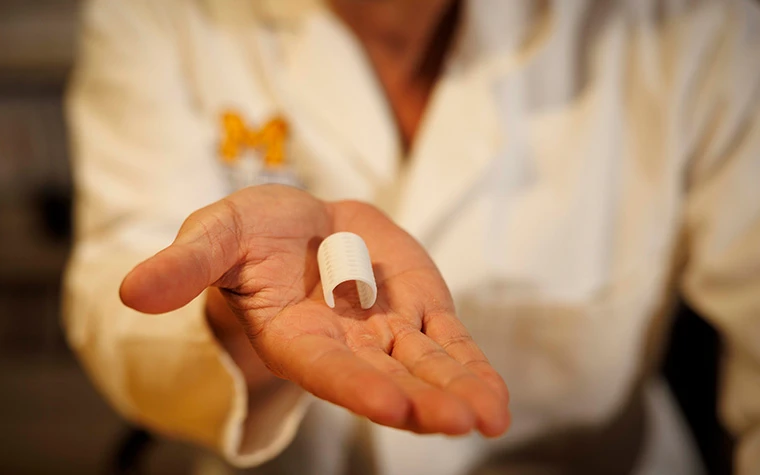
Additive Manufacturing software and service provider, Materialise, and the University of Michigan (U-M) have entered a U.S. Food and Drug Administration (FDA) pivotal clinical trial involving Materialise’s bioresorbable, 3D-printed tracheobronchial splint device.
The current clinical trial opened in January 2025 and will enroll at least 35 infants and children in an eight-year study at University of Michigan Health C.S. Mott Children’s Hospital and elsewhere. The original splint device was developed using Materialise Mimics software by Scott Hollister, former U-M professor of biomedical engineering, and Glenn Green, C.S. Mott Children’s Hospital otolaryngologist and clinical professor. The first child received life-saving treatment in early 2012.
Since then, Mimics software has been used in case planning and providing tracheobronchial splints to more than 40 pediatric patients via FDA Expanded Access (both Compassionate Use and Emergency Use) pathways. Materialise and U-M worked together to obtain an Investigative Device Exemption (IDE), held by U-M Pediatric Cardiovascular surgeon and lead investigator Dr. Richard Ohye, which has led to a clinical trial and potentially regular use of the device, if it’s approved for marketing by the FDA.
“In partnering with U-M on this life-saving clinical trial, children now have access to this ground-breaking device and procedure,” said Colleen Wivell, Director of Clinical Engineering at Materialise in a company press release. “We’ve worked tirelessly with U-M to get this project off the ground. I’m so proud to say that we are helping address an unmet need using Materialise’s best-in-class innovations. It’s about saving lives, improving quality of life, and making ongoing care more reasonable and sustainable.”
According to the company, the project’s challenges included further developing the device design, establishing the 3D printing and sterilization process, pre-clinical testing, safety and efficacy testing, obtaining appropriate regulatory approval to be able to open the trial, and establishing a clean room and good manufacturing practices. One of the team’s most significant achievements, according to Wivell, was to perform the pre-clinical testing that enabled the project to obtain the IDE.
The clinicians and engineers use the Mimics and 3-matic modules to translate anatomical data from CT or MRI for use in pre-surgical planning and preparation for 3D printing. Materialise’s method involves 3D printing polycaprolactone, a biodegradable material that is gradually absorbed into the body over years. The potential future implications of such material are obviously significant.
“Using a laser sintering process, the devices are printed right here at a dedicated Materialise facility near the U-M hospital in Ann Arbor, Michigan,” explained Wivell. “This method provides a lot of design freedom. 3D printing for hard tissue such as bone is well known; however, the potential for this bioresorbable 3D-printed material to open doors for significant advancements in soft tissue applications is immense. Materialise is at the forefront of such innovation, and we look forward to scaling up and making our innovations count.”
“This project demonstrates Materialise’s commitment to transforming healthcare,” said Bryan Crutchfield, Vice President and General Manager, North America at Materialise in the same release. “Our corporate responsibility to 3D printing and planning as drivers for a more personalized approach means that clinicians can tailor treatment plans to each patient’s unique anatomy and thereby significantly impact patient care.”