We spoke with real end users to find out how AM is used in orthotics and orthopedics.
As a digital manufacturing technology, additive manufacturing (AM) offers certain advantages for cost-effectively manufacturing unique, individual parts. For many years, rapid prototyping was the major application that made use of this capability. More recently, the concept of ‘mass customization’ has emerged, especially in the automotive and consumer products industries, such as shoes and eyeglasses.
Of course, the medical devices industry may be the industry with the strongest set of applications for customized, individual products fabricated using digital manufacturing technology. From orthodontic aligners to orthotic devices such as insoles or braces, to orthopedic implants such as knee or hip replacements and much more, additive manufacturing provides many benefits for medical device manufacturers. 3D scan data is used to create a CAD model, which can be translated 1:1 to the 3D printer, which has fewer geometry limitations compared to other manufacturing techniques, such as CNC milling. Secondly, the low lead time and on-demand nature of 3D printing allows manufacturers to meet the needs of patients quickly. These geometry and design capabilities allow designers to use complex lattice structures to deliver better performance, such as lighter weight, improved biocompatibility, and improved strength and rigidity.
To learn more about the role additive manufacturing plays today in the medical device industry, including the challenges and advantages of the technology, engineering.com spoke with Victor Phan, Orthotist and AM Application Specialist at Korthotics, an orthotics and prosthetics service based in New South Wales, Australia, and Mateo Garcia, VP of Operations at Restor3D, a North Carolina, USA-based company that specializes in personalized implants and surgical solutions.
Restor3D
Restor3D was founded in 2017 with the goal of using 3D printing technology to create better surgical devices and orthopedic implants. “The intent has always been to use additive to personalize our orthopedic offering, and giving a unique offering and best fit, instead of ‘small, medium and large,’ as you might find with our other competitors,” said Garcia. Restor3D currently uses additive manufacturing for both polymers and metals.
According to Garcia, AM provides several distinct advantages for medical implants. “Using additive, we can provide improved performance or mechanical advantages, as well as advantages to reduce lead time and inventory,” said Garcia. “AM enables us to get a solution quicker with fewer steps, to fabricate something that matches the form and function of the user, or helps the surgeon do something in a way that’s easier, cleaner, or more efficient.”
It may seem that given the advantages AM provides for both the process and end product, it would be more dominant in the medical devices manufacturing space, but Garcia notes that this is not the case. According to Garcia, the majority of orthopedic implants manufactured in the United States are produced using subtractive manufacturing processes, such as milling, and customization remains rarer, with many implants available in off-the-shelf sizes, not unlike shoes or clothing. “When companies do use additive, it’s often for low-hanging fruit, or even serialized production,” said Garcia. For a company to be fully dedicated to both AM technology and custom devices is unique, but AM continues to emerge as a technology in the industry.
Korthotics
Korthotics is an orthotics and prosthetics service based in New South Wales, Australia, operating since 2004. “For us, it’s always been about patient focus, about evidence based practice, about working with the health team, medical team, and all that in order to achieve certain outcomes,” explained Phan.
In 2017, the company began pursuing AM technology to provide a new way to fabricate medical devices, including pediatric braces and surgical guides and templates, for example.
“I like to compare orthotics as the blacksmiths of the medical industry,” said Phan. “We’re very hands on, with a technical focus, and we translate the functional needs of the medical prescription. That’s always been sort of our role in this space. A surgeon or doctor will come to us and say, “How do we get this patient in this position to achieve such and such outcome?”
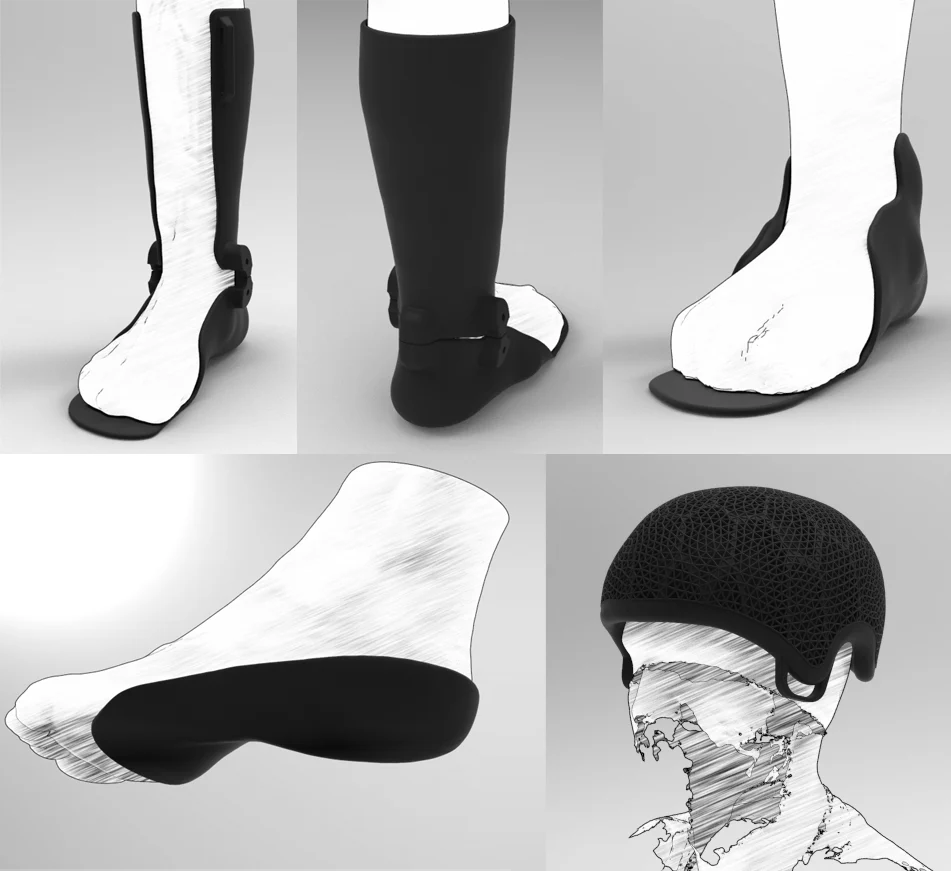
For Korthotics, the benefits of AM have been centered around flexibility and freedom of design. “With our SLS machine, we print cranial helmets for people with brain injury requiring protection in the medical space, such as epilepsy,” explained Phan. “We also print cervical collars for people who lack stability and other neck issues. We use the SLS machine mainly because these parts are very intricate, with lots of overhangs, it’s a difficult thing to print unless you use SLS. For the helmet, we’ve made it in a way to reduce impacts, so utilizing the freedom of design, lattice structures, all those kinds of things have been extremely beneficial for us.”
Challenges
Regulatory compliance
Both Garcia at Restor3D and Phan at Korthotics noted regulatory compliance as the most significant challenge to their work today. In the USA, medical devices are subject to numerous tight regulations from the FDA, and new materials and processes such as those used by AM don’t have the precedent required to speed that process. Working toward regulatory approval, according to Garcia, is a long process. “We absolutely have a regulatory burden,” he said. “The only other industry that comes close, I think, might be aerospace. The challenge with these high-risk industries is the high burden of proof that’s required of us, and we partner with businesses to develop strategies that include the regulation components, not just the devices but the methods we make the parts.”
While a material such as titanium or PA11 may already have approval, the powder form of it, for example, is completely new from the regulator’s perspective. “That’s where the FDA has been particularly cautious, and rightfully so,” explained Garcia. “I think we needed to establish a baseline, and that baseline has now been mostly set up. There are partners within the FDA that are focused on the regulation and the support for our industry in particular and for AM in particular. We’re making some headway there, but the harmonization of how we validate, the harmonization of how we clear devices, how we fabricate is still not fully there, and that speaks to the early stages of the maturity of AM in the industry,” he continued. “We need more history [of 3D printed orthopedic implants] in the body, and the only way to do that is to put more out there.”
In Australia, for Korthotics, the regulatory requirements are similarly challenging. While the requirements for orthotics, which does not include implantable devices, may not be as stringent as devices which will be implanted in the human body, Korthotics still faces these challenges, especially as AM remains a new and emerging technology. “Regulatory is slow to catch up, the engineering and materials has been pushing forward like a freight train, and regulatory has been slow to catch up,” said Phan. One strategy for the company is to work within the constraints of existing approved materials, rather than work on new approvals.
“It costs money to reevaluate materials from other industries, so I think many medical device manufacturers will say, ‘we’re going to take a step back and look at what is currently possible, then make business choices around that.’.”
An important facet of this regulatory challenge is the fact that materials, which must meet various regulatory standards, are controlled by AM vendors. In some cases, a change to the formula or a new product release can cause significant problems for medical device manufacturers.
“Being in the medical space, we need to have that reliability continuing all the way into the future,” explained Phan. “We can’t have manufacturers changing materials on us, saying, ‘Ah look, we’ve come out with a better version!’ When we’ve spent 12 months validating, we can’t flip so fast just because you tell us. We have to go through an entire case study. On paper the new material may be better, but we’re putting it on real people.”
At Restor3D, the team has faced similar setbacks. “Yes it is challenging,” said Garcia. “I think that’s where it’s so important to partner with good suppliers with good supply chain. This is where Formlabs has been a great partner for us. For example, they have invested in ISO 13485 (medical devices) accreditation for their polymer site. That extra level of scrutiny is needed for those materials.”
As much as change can be challenging, companies like Restor3D and Korthotics do want materials to improve and evolve. So, according to Garcia, the solution is clear communication between vendor and customer.
Education and awareness
Another hurdle for AM-focused medical device manufacturers is awareness and education within the industry about the advantages of additive manufacturing for the specific use case. AM vendors and medical device manufacturers both have a part to play in educating surgeons, hospitals, and even patients about these benefits.
“Ultimately, we are getting paid by the customer through the insurance, which goes through the hospital. So we have a hospital to fulfill, a surgeon to satisfy, and a customer whose life we have to help get back to normalcy, so we have a ‘multifaceted’ customer,” explained Garcia. “When we expand our business, we mainly focus on educating the medical practitioner, but It’s very important to also educate the end user who the device will end up in.”
“Education is an industry problem,” said Garcia. “And I use the word problem very intentionally: it creates a challenge if there is a better way of doing something and, as a patient, you’re getting something that is subpar or provides lower outcomes.”
For Korthotics, education has also become an important part of the journey. “One thing that we didn’t foresee is the amount of doors opening, all these companies wanting to learn what’s going on. I’m now a guest lecturer at one of our universities purely on 3D printing,” said Phan. “I give them broad strokes on the scanning, the CAD workflow, and getting the students familiar with the materials, how it reacts, these are the things you need to consider on this pathway.”
In addition to literal education at higher education institutions, Phan also provides demonstrations for medical practitioners. “Even our local health district, when we provide these devices, we’re educating them on how you don’t need a cast. We now scan: the turnaround is quicker, we don’t need more labor hours, I can work on the CAD, quicker turnaround, less invasive and traumatic. That’s the nature of digitizing, you don’t need to be as hands on. In the past that has been traumatic, necessary, but a lot cleaner workflow with scanning.”
Regional availability
Being based in Australia, Korthotics faced unique challenges in adopting AM simply because many vendors are based in the USA and Europe, and due to demand or other limitations, didn’t provide sufficient support or product availability to the Australian market, despite making some machines or systems available for sale. “We faced massive learning curves, there wasn’t anyone to tell us about those at the time,” explained Phan about the beginning of the journey, back in 2017. “To obtain a lot of the information, you’d have to go overseas and explore. In Australia, it was quite challenging because of the isolated nature of the country. We also faced other issues because of chain supply. “Trying to get information, assistance, decent competitive costings into Australia has been the biggest challenge, and the thing for us to consider as far as how to approach 3D printing into the future.”
Even as the company grew and became a leader in AM in the industry within the region, this remained an issue. “For example, when I was looking into printing TPU for foot orthotics, I went to the highest known company at the time doing the best quality product for their printed outsoles for footwear, I asked them if they provide to Australia,” said Phan. “‘No, we don’t have the service, we don’t have the supply chain, the expertise to service the machines, all these other requirements. You can buy the machine, but we can’t help you apart from Zoom,’ said the vendor.
Even when materials are available, shipping costs are prohibitively expensive at low quantities. “Unless you actually have a company willing to bring that level of servicing into the country, It’s just going to be hard for anyone to get into those high end technologies.” said Phan. It’s one main reason why the company has invested in FFF technology for many applications, as the technology is more accessible.
Looking into the future for AM in medical devices
A common prediction for 3D printing technology is the vertically integrated print station concept, where your local shoe store or cell phone case kiosk has an on-demand, automated 3d printing capability, providing custom products as easily as a paper printer can translate the digital into the physical. However, according to Garcia, it’s doubtful that this approach will supplant medical device manufacturers like Restor3D in the field of orthopedics.
“It is happening to some degree in Dental,” said Garcia. “For Orthopedics, it’s tricky because I don’t think it’s the right solution for every approach. A lot of medical facilities won’t want to take on the responsibility of being the fabricator, and you need more space than you think for a fabrication shop, including postprocessing, for example. For smaller projects such as cut guides, anatomic models, and things of that nature it is possible, but I haven’t seen it with implantables,” said Garcia.
According to Garcia, AM may never completely supplant traditional manufacturing processes, such as milling, for implantable medical device manufacturing. Instead, these technologies will continue to grow as a tool in the toolbox alongside other manufacturing techniques. “For example, AM is not set up today for a ground surface, or a polished surface,” he explained. “We use other tools or pieces of equipment to get to that surface. Whether it’s a mill or a cleaning station, the combination will always be needed.”
At Korthotics, the company looks to the future to expand and continue to build on AM capability. “In what we’re doing, predominantly large devices for the spine, a combination of FFF and FGF pellet printing will be our ideal solution. We don’t see SLA and SLS as being viable for big objects. There are too many issues with liquid vats or powder management, for example. For our direction and niche market which is going to be mainly spine, it’s going to be pellet printing using polypropylene in some kind of gantry system, so we’re not limited by space, post processing, and obviously the material has already been established. We use polypropylene over the past 30, 40 years, so we don’t have to reinvent the science behind it or validate it, we don’t need to prove it to the medical field.”
In any industry, breaking new ground or pursuing a new, emerging technology to achieve a better result always comes with challenges. But in the medical device field, as Korthotics and Restor3D demonstrate, additive manufacturing is well worth it.





