TrueDent-D resin is now available in major European markets that require CE marking.
EDEN PRAIRIE, MN and REHOVOT, Israel, Jan 15, 2025 – Stratasys has declared that its TrueDent-D resin is available for sale in Europe as a CE Mark Class I medical device. This milestone enables the sale of its TrueDent monolithic multi-shade digital denture solution in European countries that require CE marking.

Following its successful debut in the United States in 2023, TrueDent is now set to deliver a scalable, efficient, and high-quality solution for denture production for dental labs and clinicians across Europe. Interest in the TrueDent-D resin is already strong, with more than 30 customers committed to onboarding in Q1 2025.
According to a recent iData report1, the demand for denture solutions in Europe continues to grow as the region’s opportunity for dentures is projected to expand from USD 2.19 billion in 2023 to USD 2.45 billion by 2028. The TrueDent denture solution by Stratasys is designed to answer this demand with a fully digital workflow that enables dental labs to produce customized dentures efficiently, offering dentists and patients an improved outcome in fewer appointments. Stratasys estimates the total cost for a lab to produce a TrueDent denture is less than half of those produced by traditional means. One Stratasys J5 DentaJet printer can produce more than 30 full monolithic, multi-shade dentures per print job, a figure unmatched in the industry.
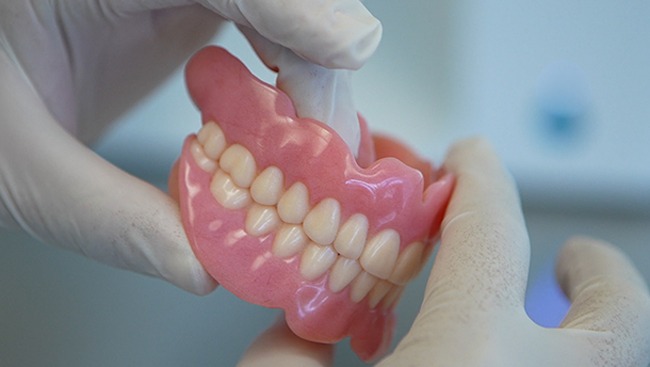
A key feature is the ability to print a duplicate denture with a click of a button enabling clinicians to quickly provide a spare or a backup for their patients. TrueDent is the only solution in the world that can produce identical dentures when needed, offering not just fit, form and function, but also aesthetics. This capability creates potential for new business models, allowing customers to scale production without increasing costs or labor requirements.
“The TrueDent solution has transformed our denture business,” said Tra’ Chambers, owner of Express Dental Laboratories, a leading U.S. TrueDent customer. “The streamlined digital workflow and precision allows us to produce up to 225 dental appliances per day while delivering high fidelity, highly aesthetic appliances to our customers. The Stratasys TrueDent offering has elevated the level of care we can provide, saving time and costs for both clinicians and patients.”
Edentulism, the condition of being without natural teeth, affects over 267.5 million people globally, including a prevalence rate exceeding 10% among adults over 50 in many regions. The percentage of denture cases produced using digital methods in the U.S. has risen from 5% in 2022 to 11% in 2024. Similarly, European dental labs are increasingly adopting digital workflows to address labor shortages and meet patient needs faster and with greater accuracy.
The TrueDent denture solution elevates the challenges faced by labs that are limited by current fabrication methods that often involve the need for manual assembly by skilled craftspeople. TrueDent is already proving its value in the U.S., where it has transformed the way dental labs operate. By implementing an unattended streamlined digital workflow, TrueDent eliminates 27 or more manual touchpoints per print, reduces chair time for dental professionals and patients, and enhances patient satisfaction with high-aesthetic, true-to-design fit, form and function appliances.
“We are thrilled to bring TrueDent-D to Europe,” said Erez Ben Zvi, vice president of healthcare at Stratasys. “Our monolithic TrueDent denture solution combines high fidelity, aesthetics, and production scalability, while reducing labor costs and enabling exact reproductions. There is growing excitement across the region for this innovative solution, which not only improves the experience for dental professionals but also elevates the standard of care for patients.”
For more information, visit stratasys.com.
1 iData Europe Market Report Suite for Dental Prosthetics, February 2024